Fig/Fig10-1.jpg)
Chapter 14 Immunologic Tolerance
1. Immunologic tolerance
--> immune system randomly generates a vast diversity of antigen
specific receptors and some of these will be self-reactive,
tolerance prevents reactivity against the body's own.
- Definition of IR and immune tolerance
Fig/Fig10-1.jpg)
- induction of immune tolerance
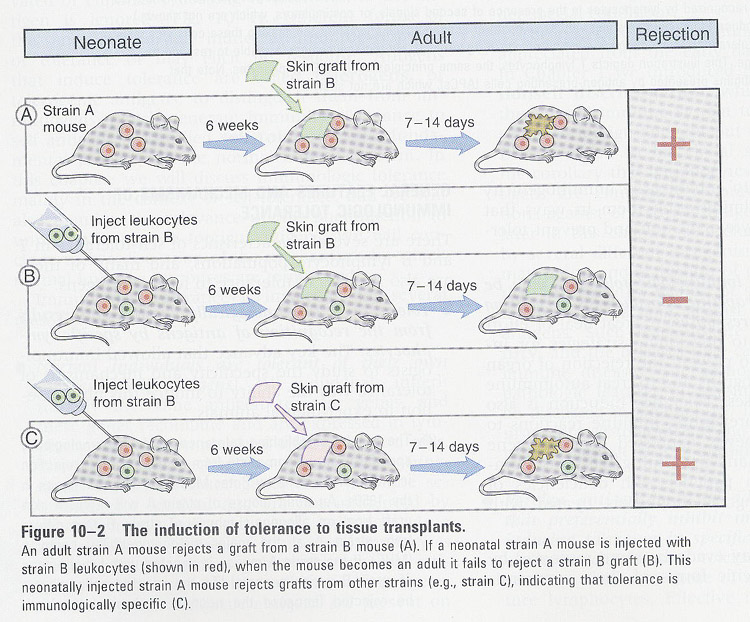
Topics: 1) mechanism by which various
Ags inhibit immune response
2) factors
that determine the nature and magnitude of responses
Importance of the elimination
of Ag itself:
in self-regulation of the immune response
1) products (antibody,
cytokine) have a short lives
2) they are secreted
for a brief period
3) effector cells
(lymphocytes) are short-lived
2. Immune tolerance
--> tolerance
results from the interaction of Ags with Ag receptors on lymphocytes
Antigens: 1) tolerogens - induce tolerance
(ex)
all self-antigens, some foreign antigens
2)
immunogens - generate immune response
(ex)
some foreign antigens - depend on physicochemical form, dose, route of administration
Role
of antigen:
1) protein Ags;
humoral and cell mediated
polysaccharide/lipids;
no cell mediated
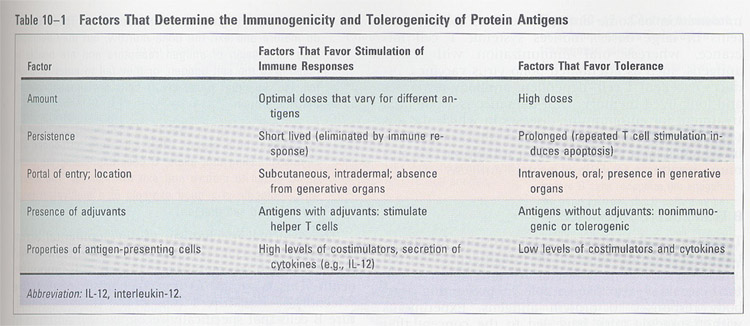
2) amount of Ags;
high dose/repeated administration of small amounts of Antigen (inhibitory)
3) methods of administration;
(table 11-1)
subcutaneous/intradermal
--> immunogenic
intravenous/oral
--> unresponsive
General
features:
1) immunologically
specific -- deletion/inactivation of Ag-specific T and B cells
2) tolerance to
self is learned/acquired
3) immature lymphocyes
are more susceptible
4) mature lymphocytes
are also susceptible under inadequate conditions
--
immunization conditions
Mechanisms: 1) deletion -- cell death of immature lymphocytes
interacted with Ags
2)
anergy -- inactivation of lymphocytes
Sites of immune tolerance;
in generative lymphoid organs (central tolerance)
in peripheral lymphoid organs (peripheral tolerance)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-014-f001.jpg)
(A)
T lymphocyte toleranc(Fig 10-5)
--> effective way for
maintaining long-lived unresponsiveness
(a) T helper cells are critical for cellular and
humoral responses
(b) low levels of Ags are sufficient to induce
the tolerance
--> mechanisms: T cell anergy
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-014-f004.jpg)
(1) clonal deletion; for self-reactive clones
-
during maturation in the thymus
-
most effective to protein Ags present in thymus
(2) clonal anergy; T cells recognize APC without the costimulators,
and unresponsive to subsequent same Ag stimulation
-
in peptide-MHC complex on synthetic lipid membrane
in
chemically treated APC
in
CTLA-4:B7 interaction
-
administration of large doses of Ags in aqueous soln's without adjuvants
- Role of costimulators in T cell anergy;
Fig/Fig10-6.jpg)
- Induction of T cell anergy using transgenic mouse (Fig 11-5)
Fig/Fig10-8.jpg)
** Activation-induced cell death
Fig/Fig10-9.jpg)
Question: what is the mechanism for tolerance to self-polysaccharides and lipids ?
(B)
B lymphocyte tolerance
- a potent tolerogen -> polysaccharides (not processed)
--> mechanisms:
(1) clonal deletion; a minor role
-
occur in B cells of bone marrow (IgM B cells)
-
evidence;
transgenic
mice of heavy and light chain genes specific H-2K(k) haplotype
-->
other haplotype mice, produce anti-transgenic antibody
-->
same haplotype mice, no anti-transgenic antibody and reduction of B cells
(2) clonal anergy;
-
by Ag-induced block in mIgs (=clonal abortion)
-
by functional inactivation
-
evidence;
a)
treatment of anti-Ig antibody on immature and mature B cells
-->
in immature B cells, endocytosed but not expressed
-->
in mature B cells, endocytosed and expressed
b)
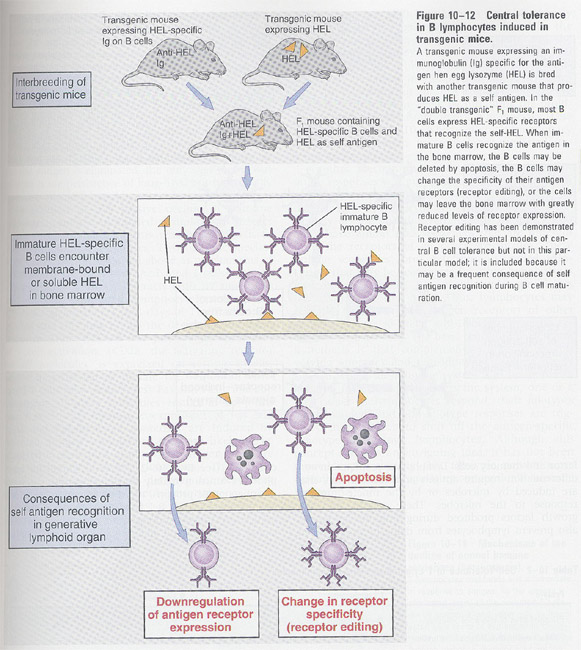
transgenic mice with HEL/anti-HEL antibody
-->
clonal anergy is short-lived and required the continuous exposure to Ag
-- Mechanism; downregulation of receptors
changes of receptor specificity
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-014-f008.jpg)
B cell tolerance in a transgenic mouse model (Fig 11-10)
(C)
Suppressor(= Regulatory) T lymphocyte tolerance
How was the suppressor T cells demonstrated ? (Fig 11-3)
--> suppressive dose: high dose of aqueous Ag administration intravenously
--> Thy-1 lymphocytes
Roles; 1) prevent immune response to
self-Ag
2) inhibit immune
response to foreign Ags
-- action mechanism of regulatory T cells
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-014-f006.jpg)
Fig/Fig10-11.jpg)
General
features;
1. induced by same conditions that induce clonal anergy
-- high dose of protein Ags, hapten w/o adjuvant,
intravenous administration
-- direct interaction with Ags w/o MHC and APC
2. CD4 positive, high level of IL-2R α chain, FoxP3
required
3. Ag-specific (?)
4. MHC role in the development of suppressor T cells is unclear
5. mediated by secreted proteins
-- active secreted form of TCR
Problems;
1. purification of the cells
2. establishment of stable cell lines or hybridomas
Inhibitory
mechanism;
1. produce an excess of cytokines with inhibitory function
--> TGF-β, IFN-γ , IL-10
2. absorb necessary growth factors
--> IL-2
3. by secreted TCR
Chapter 12 Cytokines
A. General features
① produced by innate and adaptive immunity (Fig 12-1)
② secreted for a brief time; unstable
③ produced by diverse cell types
④ pleiotropism (Fig
12-2)
⑤ redundancy (Fig
12-2)
⑥ influence on the synthesis and action of other cytokines
⑦ autocrine, paracrine, endocrine
⑧ receptor expression; regulated by other cytokines and cyokine-receptor binding
⑨ cellular responses are slow
⑩ growth factors
Fig/Fig11-1.jpg)
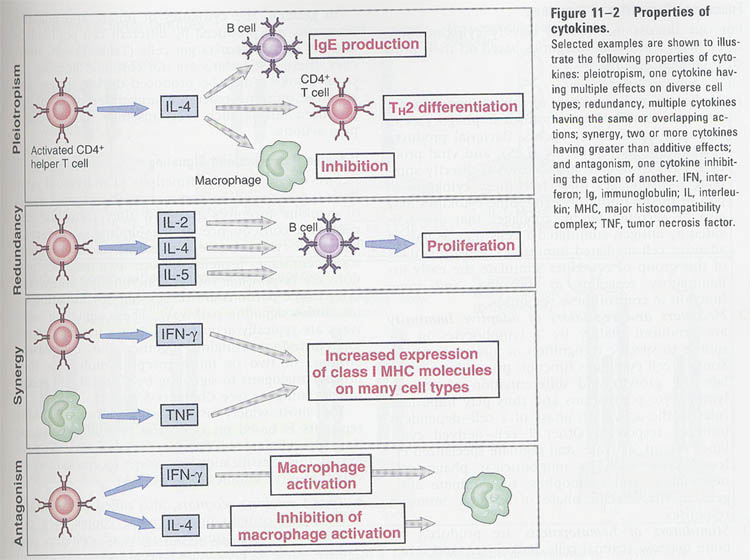
B. Cytokines
(1) type I-IFN (α, β-IFN);
same receptor and cellular responses
① α-IFN (leukocyte interferon)
--> 20 related, family
--> mononuclear phagocytes
② β-IFN (fibroblast interferon)
--> a single gene
--> fibroblast
functions
--> inhibition of viral replication
--> " of
cell proliferation (ex: Trp synthesis)
--> activation of NK cells
--> MHC I (increase); lysis by CTL, MHC II (decrease)
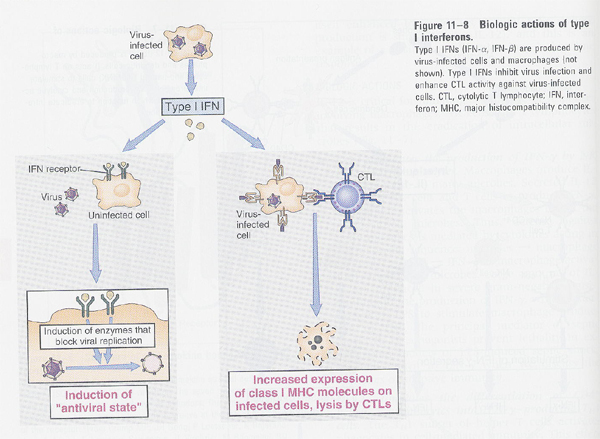
(2) type II-IFN (γ-IFN)
--> homodimeric
--> sources: T cells, NK cells in T cell deficient animals
--> enhanced by IL-2
functions
--> anti-viral activity
--> activation of phagocytes, neutrophils,
NK cells
--> MHC I and II (increase)
(3) TNF
--> host defense against gram(-) bacteria
--> sources: LPS-phagocytes, Ag stimulated T and NK cells
γ-IFN has an additive effect
--> type II protein
--> TNF
receptor is present on most of
cells
functions;
--> inflammation; 1) activation of inflammatory leukocytes,
phagocytes
2)
VED adhesive for leukocytes; by release of chemokines
--> anti-viral effect
--> fever induced
--> acute phase response (hepatocytes)
--> suppression of BM stem cell division (immunodeficiency)
--> cachexia; by long-administration
wasting
of muscle and fat cells
Fig/Fig11-5.jpg) Fig 12-5
Fig 12-5
lethal effects (at high concentration)
1) reduction of tissue perfusion (by anti-myocardial
contractility)
2) increase of blood pressure; by thrombosis
3) metabolic disturbance; glucose decrease in liver due
to the overuse of glucose in muscle
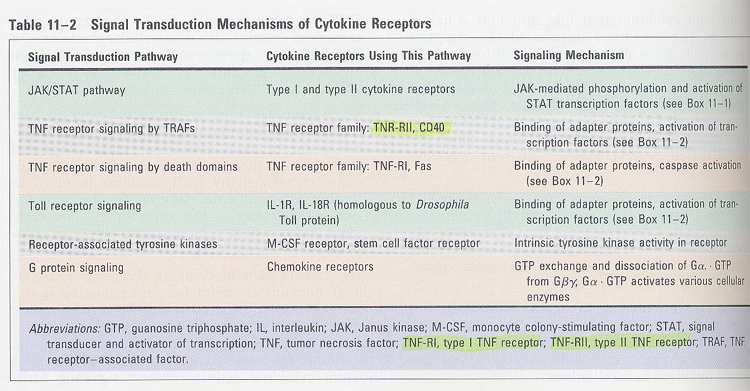
Signaling by TNF receptors (Box 12-1)
1) signaling intermediates (TRAF, RIP) ---> AP-1 and NF-kB
2) FADD (Fas associated death domain) ---> caspase-8 ---> apoptosis
** TRADD(TNF receptor-associated death domain), RIP(receptot interacting protein), TRAF(TNF receptor-associated factor)
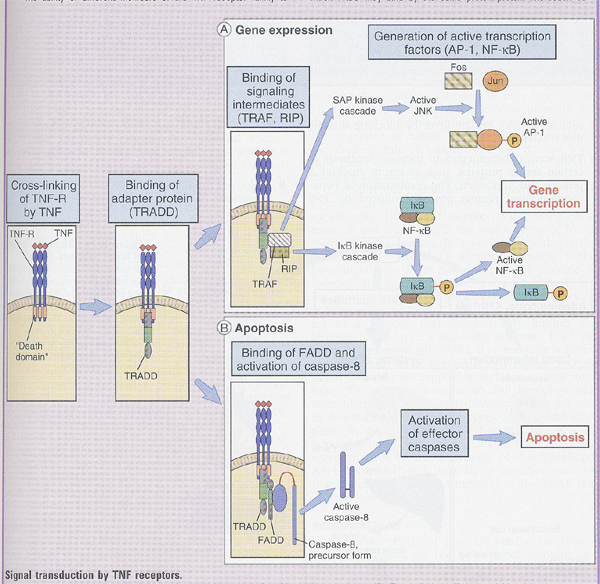
(4) Lymphotoxin (TNF-β)
--> 30% homology, but same receptor
--> sources: only activated T cells
(5) IL-1
--> sources: activated phagocytes
difference (with TNF); made by many diverse cell types
--> 2 kinds (IL-1α, IL-1β)
1) less homology
(30%<)
2) same
receptor (in most cells, IL-1β is higher),
biological
actions are identical
functions;
--> stimulation of B and T cell's growth
--> inflammatory response
--> compared to TNF
1) similarity; fever, acute
phase response, cachexia
2) difference; not lethal, no
increase of MHC expression
(6) IL-2
--> T cell growth factor
--> sources; activated CD4+ T cells
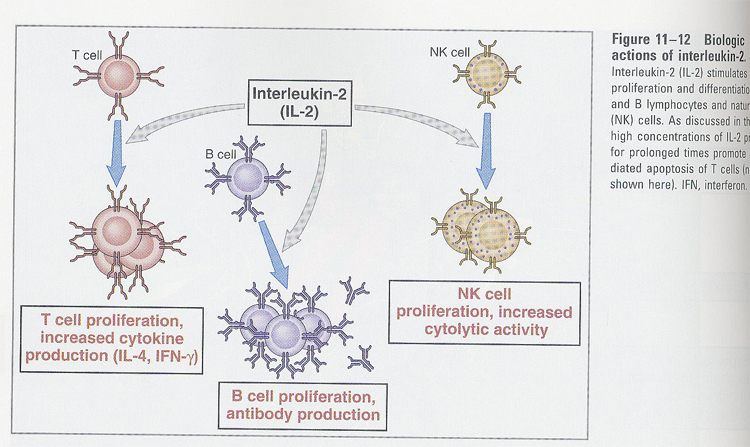
functions;
--> autocrine growth factors for B and T cells
--> stimulation of NK cells
--> IL-2R (p55 + p75)
p55 (IL-2Rα) <
p75 (IL-2Rβ) < p55 + p75
p55 (IL-2Rα) is induced after T cell activation (Fig 11-11)
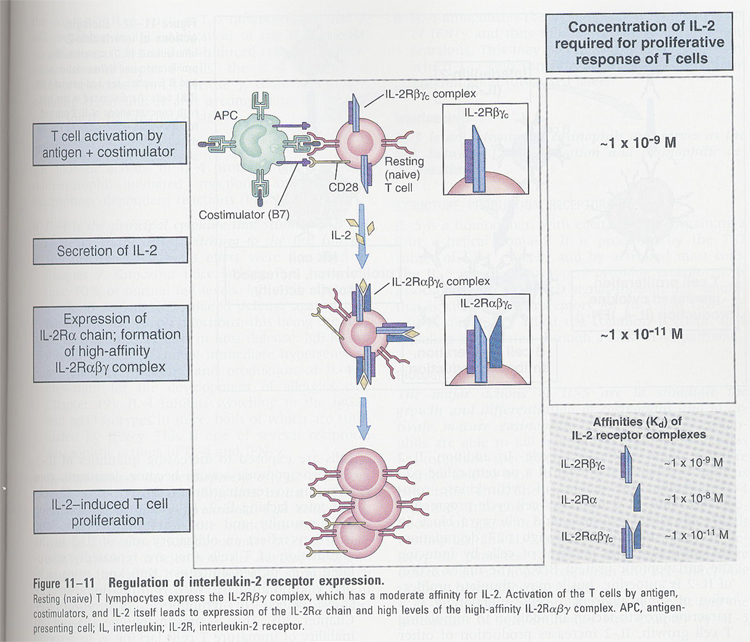
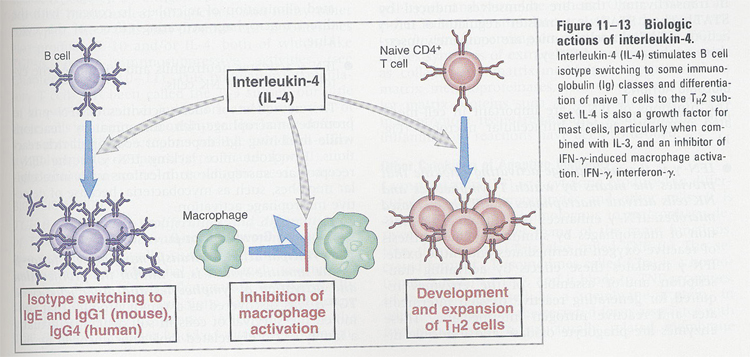
(7) IL-4
--> sources; CD4+ T cells
functions;
(Fig 11-13)
--> B cell and mast cell growth factor
--> IgE switching
--> increase of CD23 (FcR of IgE)
--> stimulation of Th2 type T cells
--> inhibition of macrophage activation
Fig/Fig13-6.jpg) ** Th1 and Th2 type cells
** Th1 and Th2 type cells
(8) IL-7
--> sources; BM stromal cells
functions;
--> committed to the B lineage cells and maturation
of CD4/CD8 negative T cells
--> IL-7R; contains γchain of IL-2R
--> growth factor for mature T cells, in vitro
(9) IL-6
--> sources; phagocytes infected with gram(-) bacteria
or IL-1 treated
vascular endothelial cells, fibroblast
functions;
--> acute phase responses
--> B cell growth factor (in late stage)
--> hybridoma, myeloma cell growth factor
(10) IL-12
--> stimulates γ-IFN production by NK and T cells
--> stimulation of CD4+ helper T cells into Th1 producing γ-IFN
--> activation of NK and CTL
Fig/Fig11-7.jpg)
Summary: Table 11-3 and
Table 11-4
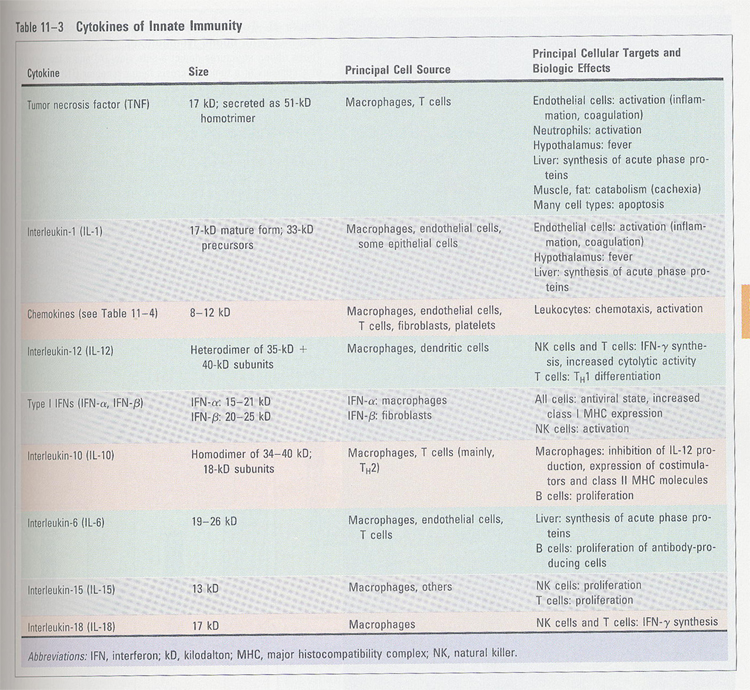 table 11-3
table 11-3
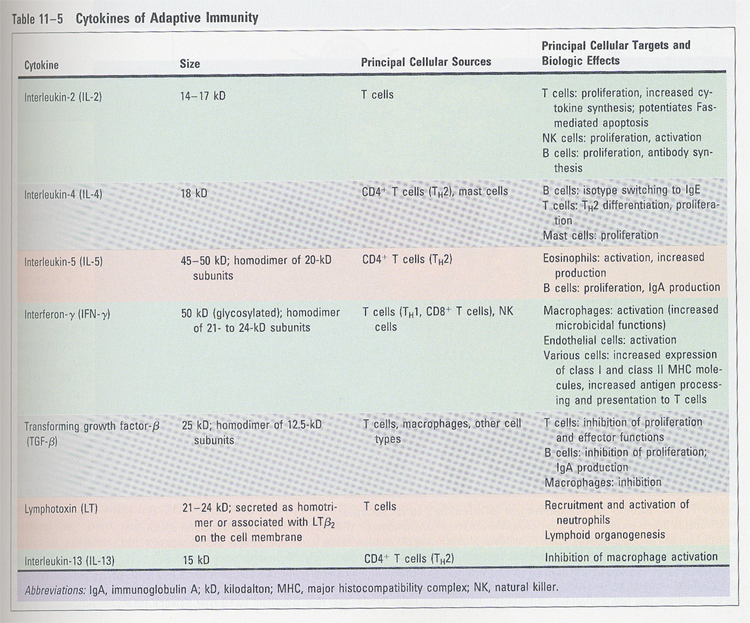 table 11-4
table 11-4
cytokine receptors and signaling
--> based on structural homologies of extracellular cytokine-binding domain
--> 5 families (Fig 11-3A)
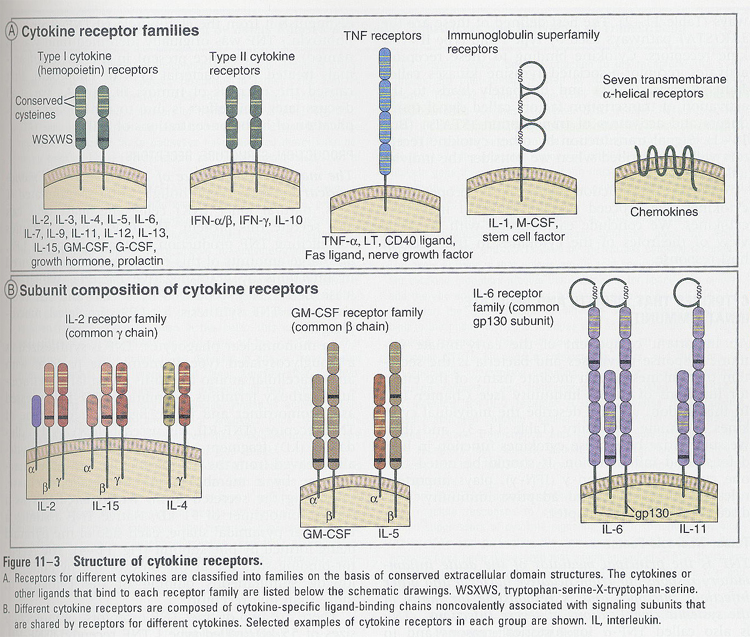
--> signaling (in type I and II cytokine receptors); Box 11-1
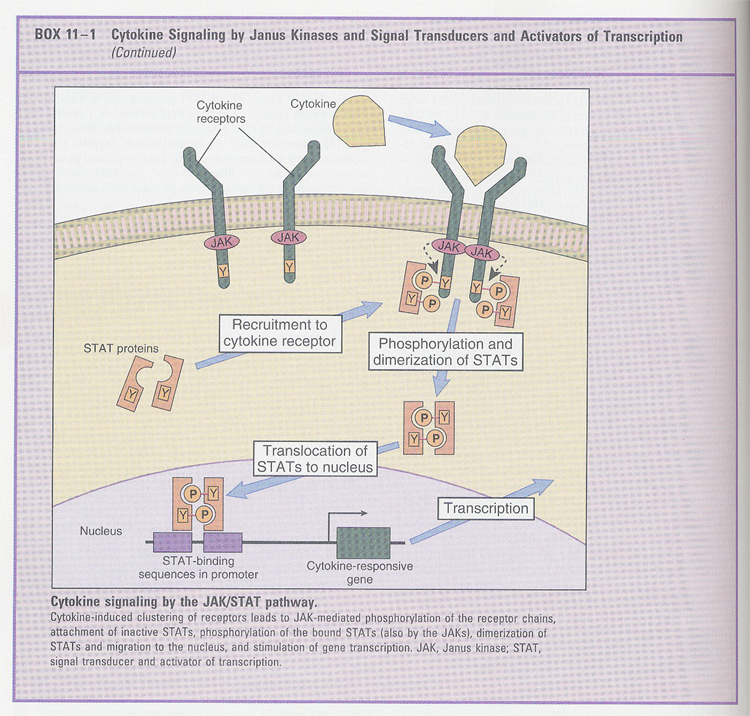
--> other signalings (Table 11-2)
Chemokines
leukocyte의 이동 (chemokinesis)을 촉진하는
cytokine들과 유사한 생리활성 물질을 지칭한다. 일반적으로 분자량이 8 - 10 KDa
정도의 크기로 대단히 작다. 이러한 chemokine들은 다시 크게 두가지 형태의 superfamily로
나누어 진다.
첫째는 CC chemokine 이며,
둘째는 CXC chemokine이다.
이들의 생리적 기능과 대표적인 chemokine들을 살펴보면 다음과 같다.
(Box 12-2); leukocyte recruitment
---------CXC---------------C----------------C--------------
----------CC-----------------C----------------C--------------
CC chemokines |
CXC chemokines |
생리적 기능 |
|
주로 monocytes/leukocyte의 이동을 촉진 |
주로 neutrophil의 이동을 촉진 |
대표적인 종류 |
|
MCP-1, HC14, MIP, RANTES |
IL-8, GRO, NAP-2, IP-10, GCP-2 |
** chemokines
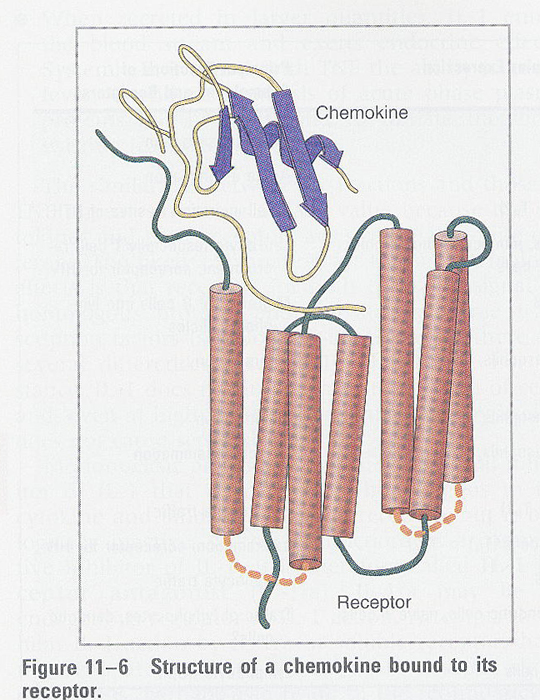
Fig/Fig11-6.jpg)
* IL-8
- 99 Aa -> 20 Aa signal seq. -> 79 Aa -> 72 or 77 Aa
; depend on cell types and culture conditions
; by N-terminal processing
- activity: N-terminal domain Glu-Leu-Arg
(ELR)
______________________________________________________________________
AVLPRSAK ELR C 20%
SAK ELR C 100%
K
ELR C 300%
ELR C 300%
LR C 3%
R C 0.1%
_______________________________________________________________________
- biological activities;
1) shape change -- contractile cytoskeleton, actin polymerization
2) exocytosis -- release of enzymes and other proteins
3) receptor for adhesion -- upregulation
4) bioactive lipids -- leukotriene
- IL-8 receptor (7 TM protein) (Fig 11-6)
1) 2 types; IL-8R1 --> for CXC chemokines and IL-8
IL-8RII
--> for IL-8
2) G protein coupled; B. pertussis treatment
3) desensitization; at high conc, by Ser/Thr phosphorylation
--> binding to the arrestin
Cytokines in hematopoiesis
Fig/Fig11-15.jpg)
Chapter 12 Complement (Effector mechanisms of humoral immunity)
-- Several biological functions of antibodies
Fig/Fig14-1.jpg)
(1) Neutralization function of antibodies
Fig/Fig14-2.jpg)
(2) opsonization function of antibodies
Fig/Fig14-3.jpg)
(3) antibody-dependent cell-mediated cytotoxicity (ADCC)
Fig/Fig14-4.jpg)
Complement system
- discovery: serum with anti-bacteria Ab
↓ heat (at 56 deg)
inhibit the lysis of bacteria
↓
suggestion
of another heat-labile component
A. biological functions
1) cytolysis; pore-forming
2) opsonization; opsonin
3) inflammation; some complement -> chemotactic
4) immune complex; clearance, solubilization
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f017.jpg)
B. pathways
1) classical pathway;
circulation - inactive form
activation
- by Ag-Ab complex
2) alternative pathway;
activation - by binding to the surface of infectious organism
MASP (mannose-associated serine protease)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f006.jpg)
Classical pathway
(1) stringent conditions
① C1 activation - CH3 (IgM), CH2 (IgG)
IgM
> IgG, aggregate
② only Ag-Ab complex, not free Ab
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f011.jpg)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f010.jpg)
(2) Ab-independent activation
① retrovirus, mycoplasma surface itself
② polyanionic molecules; DNA, heparin, chondroitin sulfate
(3) components
C1; complex (c1q + c1r(2)
+ C1s(2)) (Fig
14-9)
--> C1q binds to Ig
6
chains (3 different)
C-terminal;
globular form
--> C1s and C1r binding
both
are single chain
C1s-C1r-C1r-C1s/c1q
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f008.jpg)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f009.jpg)
C1q binding to Ig
![]()
activation of associated C1r
![]() ↘ 57KDa
↘ 57KDa
C1r (28KDa)
![]() ↘ 57KDa
↘ 57KDa
C1s (28KDa)
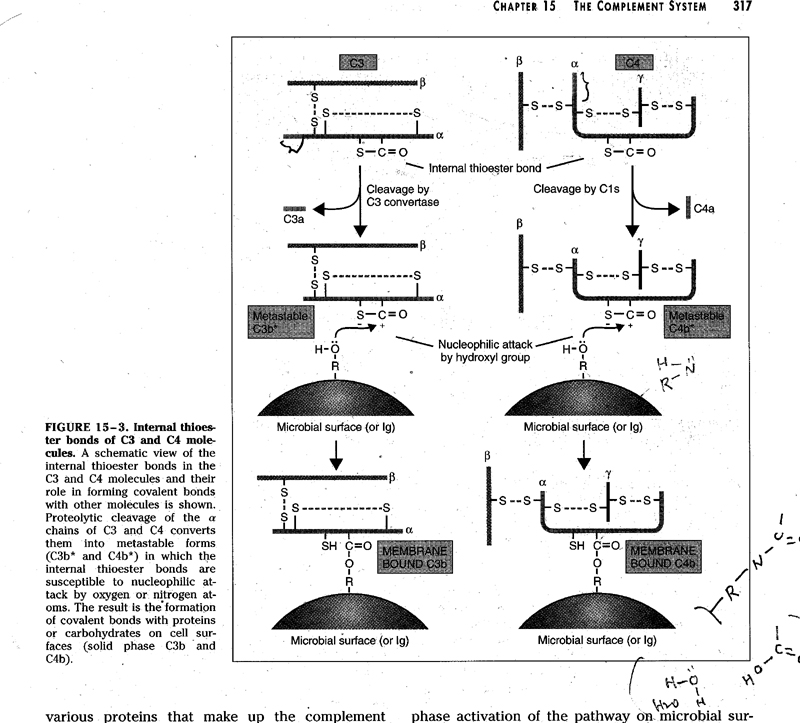
![]()
C4 + C2 * C4: 3 chains ( α,
β, γ), αchain has a thioester bond (Cys-Glu)
![]() ↘ C4a
↘ C4a
C4b (nucleophilic attack)
![]()
metastable C4b
![]()
![]()
↙ ↘
![]() water
surface
proteins (amide)
water
surface
proteins (amide)
↓
carbohydrates
(ester)
![]() iC4b ↓
iC4b ↓
attach
to the cell surface
![]()
C2
![]()
C4b.2
↙C1s
![]()
C2b
↙
C2a
![]()
C4b.2a (C3 convertase)
↙ C3:
α, βchain
![]()
C3a
↙
C3b : α, βchain
![]()
C4b.2a.3b (C5 convertase)
↙ C5
![]()
C5a
↙
C4b.2a.3b.5b
![]()
C4b.2a.3b.5b.6
![]()
C4b.2a.3b.5b.6.7
![]()
C4b.2a.3b.5b.6.7.8
![]()
C4b.2a.3b.5b.6.7.8.9 (MAC) * MAC; 1
(C5-8) + 12-15 (C9) (Fig 14-11)
reorientation
of lipid arrangement
passive
Ca2+ influx
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f012.jpg)
Regulation of MAC (Fig 14-16)
(1) HRF (homologous restriction factor), CD59
1) HRF; interfere C9 binding
2) CD59; " C9
binding
-- restriction: same species
protecting normal bystander cells from lysis
(2) Vitronectin (= S protein)
--> interfere 5b.6.7 binding to lipid memb.
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f016.jpg)
Biosynthesis
--> hepatocyte and phagocyte
Alternative
pathway (Fig 14-6)
1. in the absence of antibody
2. not autologous cells, but on the surface of microbe
3. involved proteins; factor
B, D, H, I, properdin, C3 (table 14-3)
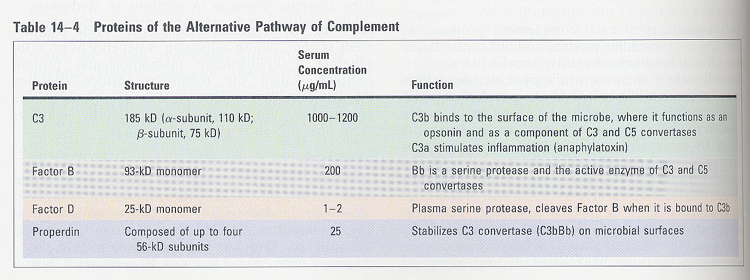
C3b
![]() ↙
factor B
↙
factor B
C3b.B
![]() ↙ factor
D (serine protease)
↙ factor
D (serine protease)
Ba
↙
Bb
![]()
C3b.Bb (= C3 convertase)
![]() ↙ properdin
(4 identical subunits, non-covalent)
↙ properdin
(4 identical subunits, non-covalent)
C3b.Bb (stable form)
![]()
C3
![]() C3b
C3b ![]() C3b.Bb.3b (= C5 convertase)
C3b.Bb.3b (= C5 convertase)
↘ C3a
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f007.jpg)
Regulation of complement pathway (Table 14-4)
Fig/table14-7.jpg)
(1) classical pathway
① C1 inhibitor (C1INH)
serpin (serine protease inhibitor)
family
blocking of C1r and C1s cleavage (by stable formation)
** mechanism; using bait seq. (mimic
seq. of substrate)
- C1INH +
C1r or C1s -> covalent ester linkage -> block of enzyme activity
prohibit
the spontaneous activation of C1
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f013.jpg)
② C4bp, DAF(decay accelerating factor), CR1
inhibit C3 and C5 convertase formation
structurally homologous
RCA(regulators of complement activity)
family
binding to C4b (C4bp)
displacement of 2a from C4b2a, Bb from C3bBb
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f014.jpg)
③ factor I
a serine protease
cleavage of C4b; ** cofactors -- CR1, C4bp, MCP(membrane cofactor protein)
-->
lack in the infectious microbes
![]()
C4c (fluid) + C4d (memb.)
(2) alternative pathway
① factor H
inhibit the binding between C3b and Factor B
displacement of Bb from C3bBb
② factor I (Fig 14-15)
cleave the C3b **
cofactors -- CR1, C4bp, MCP(membrane cofactor protein)
![]()
![]()
iC3b
3KDa
frag.
factor
I ![]()
![]()
C3dg
C3c
(soluble)
plasmin, trypsin ![]()
![]()
C3d C3g (soluble)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f015.jpg)
Complement receptor
Fig/table14-6.jpg) table 14-6
table 14-6
(1) type I (CR1, C3b receptor, CD35)
-- for C3b
and C4b
-- SCR
(short consensus repeat); total
32 개
60 - 70 Aas
binding sites are in the second
SCR of each group (7)
ex; C4bp, CR2, DAF, MCP
-- functions;
1) inhibit C3 convertase activity
2) opsonin receptor in phagocytes (Fig 14-17)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-012-f017.jpg)
(2) type II (CR2, C3d receptor, CD21)
-- for
iC3b and C3dg
for α-IFN and EBV
-- functions;
1) B cell activation
2) trapping Ag-Ab complex in germinal centers
(3) type III (MAC-1, CR3, CD11bCD18)
-- for iC3b
-- integrin family
-- carbohydrate binding capacity
-- functions;
1) phagocytosis
2) leukocyte adhesion
(4) type IV (CR4, CD11cCD18)
-- for iC3b
-- integrin family
-- functions;
1) phagocytosis
2) leukocyte adhesion
Chap 15. Immunity to microbes (Ref; Immunology, Roitt et al.)
(1) Immunity to viruses
A. defense mechanisms
1) integrity of body surfaces
2) specific anti-viral mechanism (Mx gene - inhibit influenza transcription)
3) γ-IFN -- by increase MHC I and II or activation of NK, macrophage
**
2'5' oligoadenylate synthetase / protein kinase (Fig 16-4)
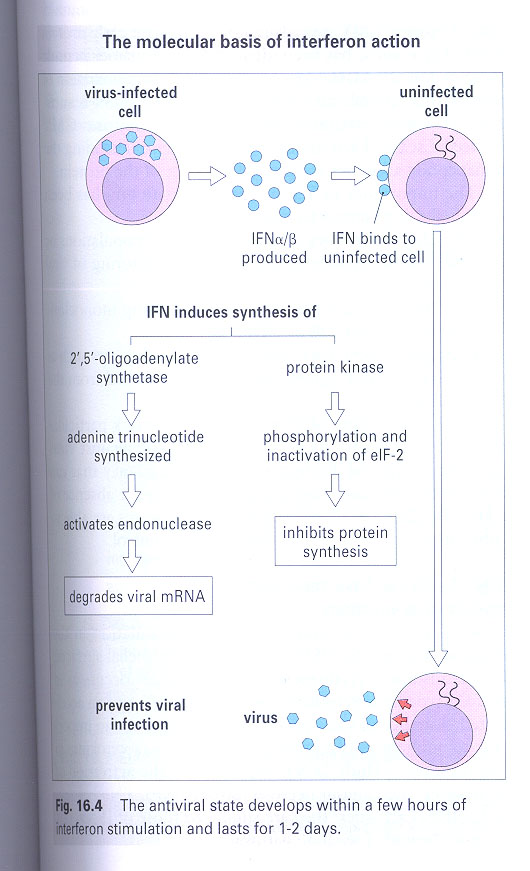
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-015-f006.jpg)
4) antibody -- neutralization
5) T cell immunity -- ① CD4 T cells ② CD8 T cells
Fig/Fig15-6.jpg)
B. evading mechanisms
1) antigenic variation -- ① antigenic drift (slight) ②
antigenic shift (radically)
2) glycoprotein -- inhibit complement activation
3) release short stretch of RNA -- interfere γ-IFN action by competition
on protein kinases
4) inhibition of MHC I transport; inhibition of antigen presentation
-- at several stages (Box
15-3)
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-015-f009.jpg)
5) release of homologous cytokine receptors or cytokines
C. Influenza virus (Fig 16-9)
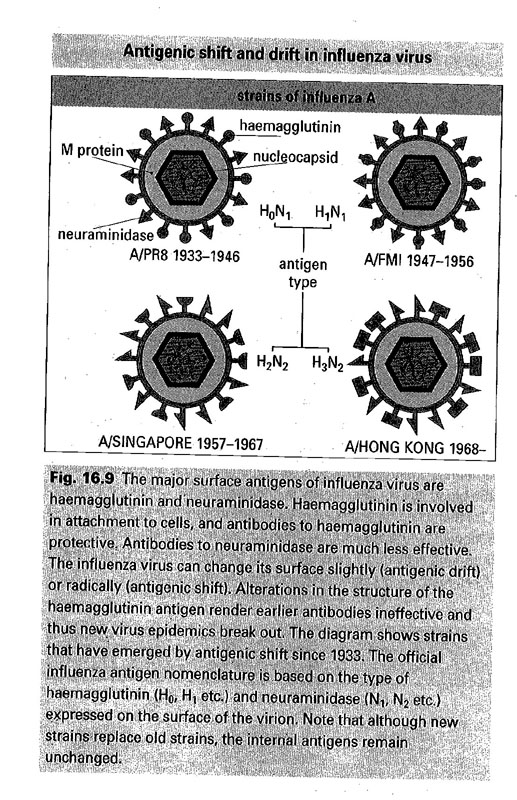
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-015-f008.jpg)
- A, B, C type
- hemagglutinin (HA) - 11 종 neuraminidase (NM) - 8 종
- 면역형/보유동물/분리된장소/분리된달/년도(HA,NM)
ex: A/swine/New Jersey/8/76 (H1N1)
(2) Immunity to bacteria
A. defense mechanisms
- depend on the structure of invading bacterias
① gram-negative -- complement, cytotoxic cells (NK,
Tc, phagocytes)
② gram-positive / mycobacteria / spirochaeta -- phagocytes
1) surface;
fatty acids in the skin --> anti-bacterial
low pH in the stomach
commensal bacterias --> colicin (anti-bacterial proteins)
2) complement activation
3) antibody
block attachment and to pick up the useful molecules
stimulation of complement activation
neutralization
** for extracellular microbe
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-015-f001.jpg)
** for intracellular microbe
/AllImagesFromCellular_and_Molecular_Immunology_7E-3/S9781437715286-015-f003.jpg)
B. evading mechanisms
(complement)
1) by outer capsule itself --> prevent complement activation
2) by membrane-bound enzymes --> degrade the fixed complements
3) by secretion of decoy proteins --> deposit of complement on decoy
proteins
Fig/table15-2.jpg)
C. killing mechanism of
phagocytes
1) O2-dependent: (Fig 17-8)
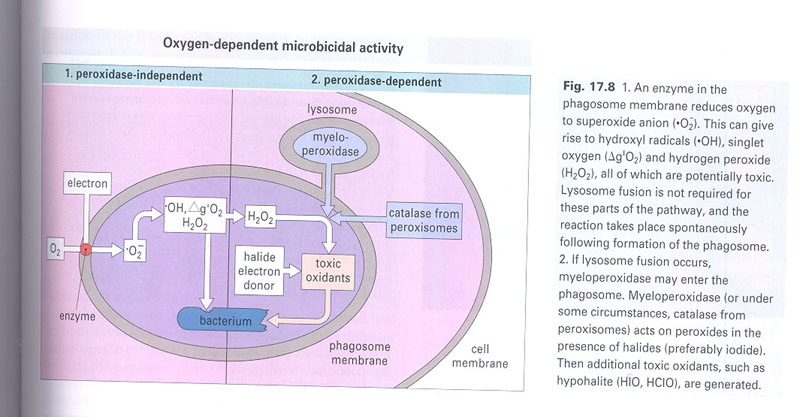
a) peroxidase independent; enzymes on the membrane of
phagosome --> reduce O2 --> oxygen intermediates
b) peroxidase of phagocytes; additional toxic oxidants
c) nitric oxide dependent; TNF/ γ-IFN --> NOS -->
NO (Fig 17-9)
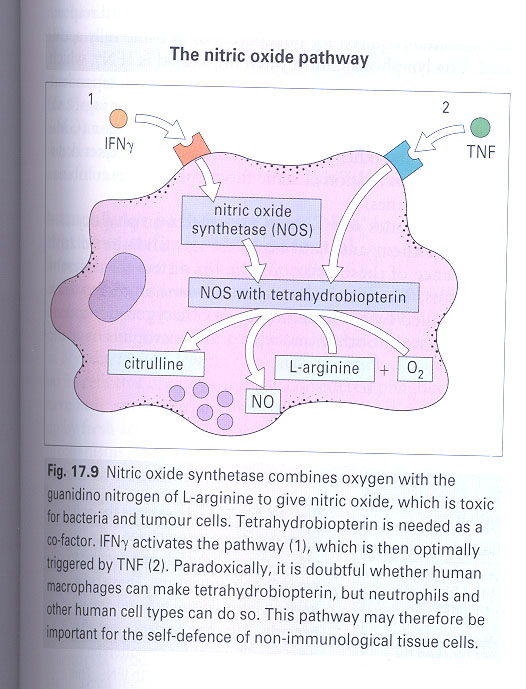
2) O2-independent:
cationic peptides --> ion-permeable channels
lysosomal enzymes
lactoferrin --> binding of Fe2+
D. evading mechanisms
(phagocytes)
1) secrete toxins --> inhibit chemotaxis
2) capsules itself --> inhibit attachment of phagocytes
3) catalase --> breakdown H2O2
4) inhibit the γ-IFN binding to the phagocytes
(3) Immunity to Fungi
A. defense mechanisms
- by cell-mediated immunity
1) TH --> cytokines --> macrophages -->
kill
2) neutrophil; in respiratory mycoses
3) defensin; a cationic protein, by formation of ion-permeable channels
4) nitric oxide
(4) Immunity to protozoa and worms
- tsetse fly; trypanosomes --> sleeping sickness / mosquitoes; plasmodium
--> malaria
A. Features of parasite
infections
① large --> a great variety and quantity of Ags
② complicate life history
③ host specificity
④ infections are chronic
B. defense mechanisms
1) macrophages, neutrophil, eosinophil, platelets
2) TH and TC
3) antibody; complement activation/inhibition of attachment/ADCC/enhancement
of phagocytosis
C. evading mechanisms
1) outer surface --> inhibit the lysosomal enzyme activity
downregulate
MHC II expression in macrophages
a
scavenger of oxygen metabolites
2) surface glycoprotein --> resemble the DAF(decay accelerating factor)
--> inhibit the complement activation
3) antigenic variation; malaria, sleeping disease (Fig 15-5)
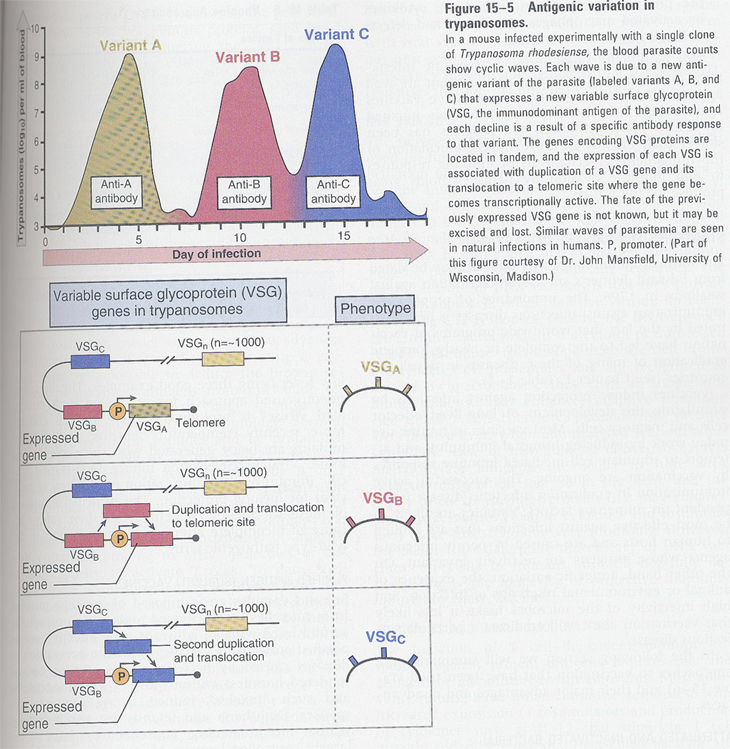
4) a thick extracellular cuticle; schistosome
5) inhibit the attraction of neutrophil; an elastase inhibitor/tapeworm
6) destruction of Ig; by proteases
(5) vaccination
A. Ags used in vaccines (table 15-6)
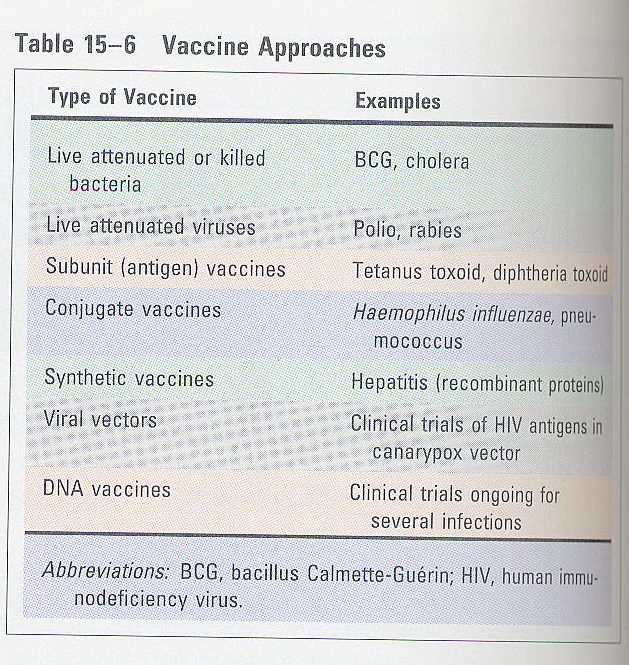
① living organisms/killed organisms; attenuated form
(ex)
BCG -- 13 yrs culture of M. tuberculosis
cholera
yellow
fever
② attenuated form; polio, rabies
③ subcellular fragments (surface antigens); safe and effective
(ex)
수막염, 임질, 간염
④ toxoids; most successful bacterial vaccines
(ex)
파상풍, 디프테리아
⑤ recombinant DNA; for small antigens
B. reason for better vaccine
using live organisms
- greater number of microbial antigens
- T cell dependency
- MHC restriction
C. vaccine safety
- contamination with unwanted proteins
- not killed
- reversion to wild type
D. difficulty to use vaccine
for parasite and worms
- polymorphic
- antigenic variation
- complicate life cycle --> different vaccines are required
Chapter 17 Tumor Immunology
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-017-f002.jpg)
Tumor antigens
종양(tumor)은 self tissue에서 유래되어지며 중요한 특징으로써 정상세포는 생성된 후 일정시기의 수명을 거친후 죽음을 맞이하나 종양세포의 경우는 무한히 증식이 가능하다는 점이다. 이러한 종양세포는 정상세포와 비교하여 여러 차이점을 보여 주는데 대표적인 예로써 정상세포와는 다른 몇몇 단백질들을 세포막에 보유하고 있어서 숙주(host)인 우리몸의 면역계가 이를 bacteria 또는 virus 같은 병원균들과 동일시 하여 즉 foreign antigen으로 인식하고 면역반응을 수행하여 종양으로의 발전을 억제하는 것으로 생각하고 있다. 이러한 개념을 immunosurveilance 라 일컫는다.
Tumor antigen이란 상기의 언급한 종양세포에 특이적으로 발현되어지는
막단백질과 같은 항원들을 지칭하며 거의 대부분은 대응하는 정상세포들의 비정상적 변이(mutation) 또는 정상적인 단백질이라도 종양세포에서의 발현조절 이상으로 말미암아 비정상적 발현이 되기 때문에 나타나는 것이다. 이러한 tumor
antigen은 virus의 단백질에 의해서도 야기 되어질 수 있으며 나아가 이러한 유발요인들은
비단백질 성분인 탄수화물 이나 지질의 비정상적인 생성을 유도하여 이러한 물질들도
tumor antigen으로써의 역할을 하게끔 하고 있다. Tumor antigen들의 발현자체 또한
대응하는 정상세포와 다를뿐 만 아니라 종양세포들간에도 상당한 차이점을 보여주고
있다.
종류; table17-1
Fig/table17-1.jpg)
(1) products of mutated oncogenes and tumor suppressor
genes
(2) products of other mutated genes
(3) overexpressed and abnormally expressed cellular proteins
(4) tumor antigens encoded by genomes of oncogenic viruses
(5) oncofetal antigens
(6) altered glycoprotein and glycolipid
(7) tissue-specific differentiation antigens
Fig/Fig17-2.jpg)
Tumor antigen을 발현의 형태에 따라 분류하여 보면 다음과
같다.
1) tumor-specific antigens (TSA)
오직 종양세포에만 특이적으로 발현되며 정상세포에는 발현
되어지지 않는다. 그러므로 숙주에 의한 강력한 면역반응을 야기시킨다.
2) tumor-associated
antigens (TAA)
종양세포뿐만 아니라 대응하는 정상세포들에서도 발현되어지며
따라서 숙주에 의한 면역반응을 야기시킬 수도 있으나 종종 숙주로 부터
self-antigen으로 인식되어 self-tolerance기작을 통하여 면역반응 자체로부터 회피할
수 있다.
Immunologic effectors
(1)
T lymphocytes
- CTL (cytotoxic T lymphocytes)를 통한 암세포의 제거는 효율성이 높다
- tumor-specific CTL; 이미 종양세포를 보유하고 있는 동물이나 사람들에서부터
동정할 수 있다
- TIL(tumor-infiltrating lymphocytes); CTL을 함유
- CD4+ T helper cells; 많은 종류의 cytokine들에 의하여 CTL development에
영향을 미치어 간접적으로는 항암작용에 분명히 연관되어 있다
TNF (tumor necrosis factor), γ-IFN를 생성분비 ->
class I MHC molecules 발현을 증대
Fig/Fig17-3.jpg)
(2)
Natural Killer Cells
- Natural killer 세포는 CTL에 의한 종양세포의 사멸기작과 매우 유사
- 종양세포들에 대한 특이성에 있어서 상당히 광범위한 점
- MHC molecules의 수가 적을수록 natural killer 세포의 좋은 표적물
- LAK cells; lymphokine-activated killer cells (IL-2)
(3) Macrophages
- Macrophage; NK 세포와 마찬가지로 Fc receptor를 보유, 항체를 매개체로
종양세포를 사멸시킬 수 있다
(lysosomal
enzymes, reactive oxygen metabolite등의 방출)
- TNF; 종양세포의 TNF receptor를 통한 세포의 사멸작용, cytoskeletal proteins의
파괴, gap junction형성의 방해 (직접
적) thrombosis 를 야기함 (간접적)
(4)
Antibodies
- 항암효과는 상당히 떨어진다
- complement activation, ADCC (antibody dependent cell-mediated cytotoxicity)기작을
이용하여 간접적으로 macrophage, NK 세포를 통한 항암작용
Tumor escape
① 종양세포들은 CTL의 주표적이 되는
MHC class I을 자체적으로 감소
② 대부분의 종양세포들은 MHC class II을 가지지 않음
③ 어떠한 종양세포들은 costimulator (예를들어 B7)를 발현시키지 않음
④ 종양세포 자체는 anti-tumor immune response를 억제할 수 있는 물질을 생성;
(TGF-β)
⑤ 종양세포들은 항원의 형태를 tolerogenic form로 표현
⑥ antigenic modulation
⑦ 종양세포들은 일반적으로 유전자 불안정성; MHC에 변이가 일어나 MHC의 고유기능인
antigen presentation 기능을 하지 못함
⑧ antigen masking; 세포벽의 glycocalyx molecules에 의한 것
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-017-f005.jpg)
Tumor immunotherapy
Active tumor immunotherapy
host의 면역반응을 증대시킴으로써 궁극적으로 종양세포를 치료하는 개념이다.
(1)
nonspecific stimulation
- 크게 3가지 정도의 방법이 이용되고 있다.
① BCG 투여방법; macrophages를 활성화, 실제로 방광암 이나 피부암환자
② 적은양의 CD3 항체를 투입; T 세포의 polyclonal activation을 유도
③ cytokines의 투여; 숙주의 면역시스템을 증대
(2)
vaccination
- 대부분에 있어서는 성공적인 결과를 얻지 못하였다
- tumor-specific antigen의 유전자를 클로닝하여 암환자에 투여하는 방법
Fig/table17-2.jpg)
(3)
costimulator 및 cytokines 처리
- cytokines을 투입하여 전반적으로 숙주 (즉 암환자) 의 면역시스템을 강화
- 결핍된 costimulator (예로써 B7 molecule)의 유전자를 transfection시킴
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-017-f007.jpg)
Passive tumor Immunotherapy
외부적으로 immune effector들을 암환자와 같은 치료가 필요한 환자에 주입하여 치료하는
방법을 말하는 것이다
(1)
adoptive cellular therapy
- 인위적으로 배양한 면역세포들을 암환자에 투입
1-A. LAK cell therapy
- 암환자의 peripheral blood leukocytes를 분리
- 인위적으로 고농도의 IL-2를 넣어서 배양한 다음 LAK 세포를 만들고
- 다시 환자에 주입하여 치료
1-B. TIL therapy
- 근거는 TIL에 항암작용에 중요한 면역세포들인 NK 세포 와 CTL이 함유
- 오직 일부분 세포들이 항암작용에서 그 기능을 수행하는 것
- TNF와 같은 뛰어난 항암효과를 가진 cytokine의 전달수단으로 이용
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-017-f008.jpg)
(2)
therapy with anti-tumor antibody
- 하나의 목표물(종양세포)에 공격무기(toxic agents)를 전달하는 하나의 수단
- 공격무기는 ricin, diphtheria toxin; 강력한 단백질 생성 억제제
- Immunotoxin; ① toxicity의 상실이 없어야 하며
②
target에 대한 특이성
③
systemic effects (hepatotoxicity, vascular leak syndrome)이 없어야함
Fig/table17-5.jpg)
Chapter 20 Congenital and aquired immunodeficiencies
Fig/table20-1.jpg)
(1) B cell immunodeficiency
- defective in Ab production
- recurrent infections with pyogenic microorganism (pneumococcus,
streptococcus)
"
intestinal
parasites
"
virus
(polio)
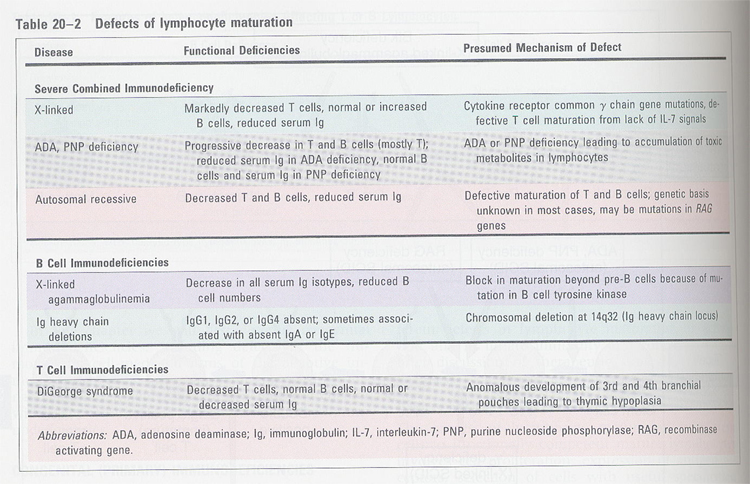
(2) T cell immunodeficiency
combined immunodeficiency
- SCID; ① adenosine deaminase (ADA) --> block DNA synthesis,
transmethylation
② purine nucleoside
phosphorylase (PNP)
③ MHC II
④ IL-2R γchain
(IL-2, IL-4, IL-7)
⑤ RAG-1 and RAG-2
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f001.jpg)
(3) Defects in lymphocyte activation
- 장소
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f002.jpg)
- 종류
Fig/table20-3.jpg)
(4) Wiskott-Aldrich syndrome
- eczema (습진), reduction of platelets
- decrease in T cells
- no Ab production response to LPS
- susceptible to pyogenic bacterias
- lymphocyte number is normal
- short arm of X-chromosome
linked disease
- defective in glycosylation
of membrane proteins
(5) Ataxia-telangiectasia
- frequent cancer with advancing age
- effect on both B and T cells (decreased T cells, normal
B cells)
- translocation in Ig or TCR loci
- defective in DNA repair
Acquired Immunodeficiency
- 종류
Fig/table20-5.jpg)
- HIV life cycle;
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f005.jpg)
- entry mechanism;
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f006.jpg)
- How HIV is progressed into disease ?
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f007.jpg)
/AllImagesFromCellular_and_Molecular_Immunology_7E-4.repair/S9781437715286-020-f008.jpg)